
Triple Negative Breast Cancer (TNBC) affects 15% of women with breast cancer, and has relatively poor outcomes due to the high level of treatment resistance. Many chemotherapy drugs work by damaging the DNA of cancer cells, but TNBC cells are often able to repair this damage, allowing the tumour to become resistant to treatment.
Professor Rob Baxter (Kolling Institute, University of Sydney) has worked for many years on discovering how cells are able to repair this type of damage, and was recently awarded a Member of the Order of Australia (AM) for his service to medical research. His team discovered that a protein known to regulate the growth of some cancer cells (IGFBP-3) could form partnerships with two proteins, NONO and SFPQ, to repair the DNA damage caused by chemotherapy. Subsequent experiments showed that if these protein interactions are prevented, breast cancer cells are more responsive to chemotherapy. This means that the same dose of chemotherapy becomes more effective at killing the cancer cells.
This effect is shown in the below images; on the left, the yellow dots inside each cell nucleus (stained in blue) show the interaction between IGFBP-3 and the NONO protein. This is actively reversing the effect of chemotherapy on the cells, and will allow the cancer cells to resist the treatment. The image on the right shows the effect of dual therapy, where a second drug, called a PARP inhibitor, is given to specifically prevent the interaction between the proteins. In this case, the proteins can no longer block the effect of chemotherapy, leading to an increase in the death of the cancer cells.
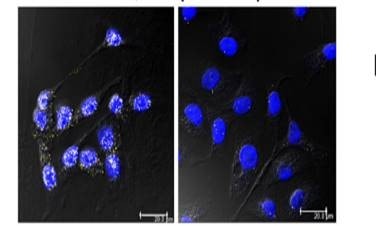
On the left, the yellow dots inside each cell nucleus (stained in blue) show the interaction between IGFBP-3 and the NONO protein. The image on the right shows the effect of dual therapy, where a second drug, called a PARP inhibitor, is given to specifically prevent the interaction between the proteins.
“This is a promising approach to treating TNBC drug resistance,” said Prof. Baxter. “Our discovery of the interaction between IGFBP-3 and the NONO and SFPQ proteins now opens up new treatment possibilities, which will improve outcomes for women with this cancer.”
More News Articles
View all News


